Electrodialysis and Bipolar Membrane Electrodialysis (EDBM) for Efficient Conversion of NaCl to HCl and NaOH: A Pilot-Scale Study
- Gu Zhouying
- Dec 10, 2025
- 16 min read
Abstract:
This study demonstrates the operation of the YASA ET pilot-scale electrodialysis (ED) and bipolar membrane electrodialysis (EDBM) system in the conversion of sodium chloride (NaCl) to hydrochloric acid (HCl) and sodium hydroxide (NaOH). The system, featuring a 10-pair Saifu EDBM stack, was operated under constant applied voltage (20 V) and controlled flow conditions. Real-time measurements of conductivity, pH, temperature, current, and energy consumption were used to monitor the process, revealing efficient ion transport and water dissociation. The acid and base compartments reached high concentrations of HCl and NaOH, respectively, validating the effectiveness of the DESALT® technology for large-scale industrial applications, including acid-base recovery and zero-liquid-discharge (ZLD) strategies. The findings highlight the scalability and reliability of the system for future industrial applications in chemical resource reclamation.
Keywords:
Electrodialysis, Bipolar Membrane Electrodialysis, NaCl Conversion, HCl Production, NaOH Production, DESALT®, Zero-Liquid-Discharge (ZLD), Acid-Base Generation, Pilot-Scale Study, YASA ET.
YASA Environmental Technology Co., Ltd.
No. 588, Xinjinqiao Road, Pudong, Shanghai, China
Email: info@yasa.ltd
Website: www.yasa.ltd
Introduction
The DESALT Pilot Equipment is a cutting-edge platform for testing and developing electrodialysis (ED) and bipolar membrane electrodialysis (EDBM) technologies. Constructed with corrosion-resistant materials, the system is designed for high durability and long-term operation in challenging environments. The pilot unit integrates precision pumps for circulating feed solutions, acid, base, and electrode solutions. It also includes flow meters and pressure gauges for real-time monitoring, as well as sampling and ball valves for easy operation and process control. A Siemens PLC system controls the entire process, while a high-efficiency DC power supply powers the membrane stack.
The system is equipped with pH and conductivity meters for precise measurement of process streams. Data acquisition, storage, and analysis are automated using Modbus-compatible data logging software. The system also includes real-time visualization and analysis capabilities through Ethernet connectivity. To ensure stable operation under high-temperature or gas-evolving conditions, the system can be outfitted with a heat exchanger and a ventilation module.
The DESALT pilot unit is adaptable, supporting both ED and EDBM membrane stacks for desalination, acid-alkali generation, and ion separation research. With reliable components and intelligent control, this system serves as an efficient platform for membrane electrochemical experiments.
Bipolar Membrane Electrodialysis Test with Pilot Skid
1.1. Feed Solutions for EDBM Test
In the EDBM experiment, sodium hydroxide (NaOH) solutions were used as the working electrolyte across all process tanks. The electrode rinse solution was prepared by diluting 500 mL of 1 M NaOH with water to create 1 L of electrolyte solution. This ensured high ionic conductivity for stable and efficient current flow within the electrode compartments.
The feed solution for acid and base generation consisted of NaCl at 100 g/L. This concentration provided the necessary Na⁺ and Cl⁻ ions for transport through the cation- and anion-exchange membranes, respectively. By maintaining stable osmotic and concentration gradients, the configuration minimized unwanted back-diffusion of H⁺ and OH⁻ ions during the electrodialysis process.
Both acid and alkali streams were continuously recirculated through their respective flow loops to ensure uniform ionic distribution, stable temperature conditions, and steady formation of HCl and NaOH throughout the experiment. This setup optimized ion transport and water dissociation, allowing for controlled conversion of NaCl into high-purity acid and base products.
1.2. Stack Configuration
The test was conducted using a Saifu EDBM stack, mounted on the YASA ET pilot skid system, featuring 10 cell pairs with a membrane size of 110 × 270 mm. Each cell pair consisted of alternating Anion Exchange Membranes (AEM), Bipolar Membranes (BPM), and Cation Exchange Membranes (CEM). The characteristics of these membranes are outlined in the following tables:
Table 1. Characteristics of the ion-exchange membranes (AEM, CEM) used in the EDBM stack.
Property | Value |
Ion-exchange capacity | 0.90 - 1.10 mmol/g |
Wet thickness | 70 - 80 µm |
Water uptake | 20 - 25 wt.% |
Area resistance | 4.5 - 5.5 Ω·cm² |
Transport number | > 0.97 |
pH range | 0 - 14 |
Temperature range | 15 - 40 ˚C |
The membranes are high-performance ion-exchange polymers with excellent chemical and thermal stability. Their low area resistance and high transport number make them suitable for efficient water splitting, acid, and alkali production in both laboratory and pilot-scale EDBM systems.
Table 2. Characteristics of the Bipolar Membrane (YSEDBM-1) used in the EDBM stack.
Property | Value |
Water Dissociation Efficiency | > 96% |
Water Dissociation Voltage | 1.0-1.3 V |
Thickness | 200-220 µm |
Applicable temperature | 15 - 40 ˚C |
IEC (ion exchange capacity) | 1.7 – 1.9 mmol/g |
Area electric resistance | 0.7 – 0.9 Ω·cm² |
Pressure | > 0.2 MPa |
Selective permeability | > 95% |
Application | EDBM for acid and base production |
1.3. Electrolyte Type
The electrolyte solution used in the electrode compartments consisted of 500 mL of 1 M NaOH diluted with 500 mL of water to obtain a total working volume of 1 L. This electrolyte concentration was selected to provide high ionic conductivity within the electrode chambers, thereby ensuring stable current flow during electrodialysis operation. The strongly alkaline environment also promotes efficient water dissociation at the bipolar membrane interface, enabling consistent generation of H⁺ and OH⁻ ions under the applied electric field.
The use of sodium hydroxide as the electrode electrolyte is advantageous because it is fully compatible with both cation- and anion-exchange membranes, avoiding the introduction of multivalent or fouling-prone species. Maintaining an alkaline electrode rinse minimizes electrode polarization and significantly reduces the risk of fouling or corrosion during continuous operation. Overall, this electrolyte composition supports stable electrochemical performance, enhances water-splitting efficiency, and contributes to the long-term operational reliability of the EDBM system.
1.4. Volume and Voltage
The electrodialysis experiment was conducted under a constant applied voltage of 20 V, delivered by a regulated DC power supply. Operating the EDBM stack at a fixed voltage ensures a stable electric field that drives both ion migration through the ion-exchange membranes and water dissociation at the bipolar membrane interface. Each of the three circulating loops, electrolyte, acid, and alkal,i was maintained with a working volume of 1 L, providing sufficient hydraulic capacity to support continuous recirculation. This configuration helps maintain uniform ion distribution, prevents local concentration polarization, and stabilizes the osmotic and electrical conditions across the membranes.
The applied voltage of 20 V was selected as an operational compromise that provides efficient ion transport and consistent water splitting while avoiding excessive Joule heating or unwanted gas evolution at the electrodes. Under these conditions, the system achieves effective formation of acid and base streams while maintaining thermal stability and protecting both the membranes and electrode surfaces during continuous operation.
1.5. Flow Rate
The flow rates were carefully controlled to ensure balanced and stable circulation:
Electrolyte compartment: 40 L/h
Acid and alkali compartments: 70 L/h each
Maintaining balanced flow rates ensured homogeneous mixing, reduced boundary layer effects, and prevented concentration polarization near the membrane surfaces. The peristaltic and magnetic drive pumps maintained constant hydraulic flow, ensuring consistent electrochemical performance throughout the experiment.
Procedure
First, the entire EDBM system was rinsed with tap water for approximately 15–20 minutes to remove any residual impurities. After rinsing, the tap water was drained from all tanks using the sampling valves. In the second step, the feed and electrolyte solutions were prepared. The feed solution consisted of NaCl at 100 g/L, while the electrode electrolyte was prepared by diluting 500 mL of 1 M NaOH with 500 mL of water to obtain 1 L of electrolyte solution.
In the third step, the DC power supply was connected to the corresponding anode and cathode terminals of the EDBM membrane stack, and the system was energized. Subsequently, the Modbus data-acquisition unit was connected to the computer to enable automatic logging of operational parameters. pH and conductivity probes were then placed in the acid, alkali, and electrolyte tanks to continuously monitor their respective physicochemical changes throughout the experiment. Measurements were recorded at 5-minute intervals, and all acquired data were exported for further analysis.
Results and Discussion
1.6. Electrical Conductivity graph
The salt conductivity exhibits a continuous decline from 105 mS/cm at the start to near 0 mS/cm by 80 – 90 min, reflecting the systematic depletion of NaCl from the dilute compartment. This behavior directly corresponds to the transport of Na⁺ ions across the cation-exchange membrane (CEM) toward the base compartment and Cl⁻ ions across the anion-exchange membrane (AEM) toward the acid compartment.
During the first 20 minutes, salt conductivity drops from 105 mS/cm to 80 mS/cm, indicating the initial, rapid transfer of ions driven by strong concentration gradients. Between 20 and 40 minutes, conductivity decreases further to 50 mS/cm, representing the mid-stage of the process in which the ion transport rate is still high but is gradually slowing as the salt concentration becomes lower. By 60 min, conductivity reaches ~10 mS/cm, showing that very few ions remain available for electrodialysis transport. Finally, by 80 – 90 min, conductivity approaches zero, marking almost complete NaCl removal from the dilute stream, and signifying the system has entered a transport-limited regime where ion depletion controls the process.
The conductivity of the acid compartment rises rapidly from 20 mS/cm to 90 mS/cm within the first 10 minutes, reflecting the fast generation of H⁺ ions at the bipolar membrane and the simultaneous migration of Cl⁻ ions from the salt compartment into the acid compartment through the AEM. Because H⁺ ions possess extremely high ionic mobility, even modest increases in their concentration drastically elevate conductivity during the early stages.
Between 10 and 30 minutes, acid conductivity increases linearly from 90 mS/cm to 260 mS/cm, demonstrating sustained and efficient production of HCl as both water splitting and anion transport proceed under strong driving forces. From 30 to 60 minutes, the acidity continues to strengthen, reaching 350 mS/cm, indicating a high concentration of HCl in the acid loop.
After 60 minutes, the increase slows, and the conductivity plateaus at ~375 mS/cm between 70 and 90 minutes. This plateau occurs because the supply of Cl⁻ from the dilute stream diminishes as the salt concentration drops, reducing the overall acid production rate. The system enters a concentration-controlled region where ion depletion limits further increases in acid strength.
Base conductivity increases from 10 mS/cm at 0 min to 60 mS/cm at 10 min, showing rapid formation of NaOH as Na⁺ ions migrate through the CEM and combine with water-splitting-generated OH⁻ ions on the alkaline side of the bipolar membrane. The increase is less steep than in the acid compartment because OH⁻ has significantly lower ionic mobility compared to H⁺, and Na⁺ diffusion is more strongly influenced by the decreasing NaCl concentration in the dilute.
Between 10 and 30 minutes, conductivity rises steadily from 60 mS/cm to 110 mS/cm, indicating continuous NaOH production driven by strong ion-migration gradients. This upward trend continues to 150 mS/cm at 45 minutes, marking the mid-stage where ion availability and water splitting are still sufficient for efficient base formation.
After 45 minutes, the conductivity gradually levels off, stabilizing between 165–170 mS/cm from 60 to 90 minutes. This plateau signifies that the rate of NaOH formation becomes limited by the declining supply of Na⁺ ions in the feed compartment. The stable high conductivity in the base loop indicates that NaOH concentration has approached its maximum possible under the operating voltage and feed composition. As we can see in the given figure below

Figure 1: The electrical conductivity profiles of the salt, acid, and base compartments during the electrodialysis process, highlighting the progressive decline in salt conductivity and the corresponding increase in acid and base conductivities over time, as NaCl is converted into HCl and NaOH.
1.7. pH Vs Time
The acid-compartment pH decreases sharply from 3.0 at 0 min to 2.2 at 5 min, and further to 1.8 at 10 min, indicating rapid accumulation of H⁺ ions generated by water dissociation at the bipolar membrane. This early-stage drop corresponds to the period of the strongest ionic driving force, when the NaCl feed still contains abundant Cl⁻ ions that readily migrate through the anion-exchange membrane and combine with H⁺ to form HCl.
Between 10 and 40 minutes, the pH decreases more gradually from 1.8 → 1.6 → 1.5, reflecting continued but slightly slower acid formation as the salt concentration in the feed begins to fall. Despite the lower availability of Cl⁻ ions, the bipolar membrane continues to supply H⁺ efficiently, and the acid loop maintains strong acidity.
From 50 to 90 minutes, the pH stabilizes between 1.4 –1.5, indicating that the acid stream has reached a near-steady-state concentration of HCl. This plateau signals that the system has entered a transport-limited region in which the rate of H⁺ generation and Cl⁻ transport is balanced by the diminishing ionic content of the dilute. Overall, the trend confirms highly effective acid production throughout the EDBM run.
The pH of the salt compartment declines moderately from 3.3 at 0 min to 3.1 at 10 min, and then more noticeably to 2.6 at 20 min and 2.3 at 40 min. This consistent acidification is characteristic of a dilute stream undergoing strong ion depletion. As Na⁺ and Cl⁻ ions are continuously removed from the salt compartment, the solution becomes progressively less buffered, making even small amounts of proton crossover from the acid loop sufficient to lower the pH.
By 80 – 90 minutes, the salt-stream pH reaches ~2.0, corresponding to near-complete depletion of NaCl. With fewer ions remaining to neutralize transported H⁺, even trace proton leakage or the natural dissociation of water under high resistance causes a significant relative shift in pH. The gradual but steady decline reflects the combined effects of dilution, low ionic strength, and the membrane’s inherent proton permeability at high transport load.
The base compartment maintains a highly alkaline pH throughout the experiment, starting at 12.3 at 0 min and fluctuating only slightly between 12.1 and 12.4 over the entire 90-minute period. This stability underscores the efficiency of OH⁻ generation at the bipolar membrane and the continuous migration of Na⁺ ions from the feed through the cation-exchange membrane.
The slight dip to 12.1 at 20 min, followed by an increase back to 12.4 at 30 min, reflects normal early-stage dynamics as Na⁺ and OH⁻ begin to accumulate and the flow loop reaches steady-state conditions. After stabilization, the pH remains in the narrow range of 12.2–12.3 from 40 to 90 minutes, indicating a strong, well-buffered NaOH solution.
The overall behavior demonstrates that OH⁻ generation and Na⁺ transport remain balanced throughout the test run, and that the system effectively produces concentrated NaOH with minimal pH drift despite changes in ionic resistance and dilute depletion

Figure 2: The pH profiles of the salt, acid, and base compartments during the electrodialysis process, showing the acidification of the salt compartment and the stable alkalinity of the base compartment as HCl and NaOH are generated, and the sustained strong acidity in the acid loop as ion migration proceeds.
1.8. Temperature vs Time graph
Temperature increases in all three compartments throughout the EDBM operation as a result of Joule heating, ion-transport resistance, and exothermic water-splitting reactions at the bipolar membrane. In the salt compartment, the temperature rises from 24.0˚C at 0 min to 30.0˚C at 20 min, reflecting the rapid establishment of current flow through a highly conductive NaCl medium. As the experiment progresses, the salt temperature continues to increase to 33.5˚C at 40 min, corresponding to increased electrical resistance as Na⁺ and Cl⁻ ions are gradually removed. The peak temperature of 36.0˚C between 60 – 70 min marks the point at which ion depletion in the diluate stream leads to maximum resistive heating. After this point, the temperature declines slightly to 35.0°C at 90 min, consistent with the sharp decrease in current once the feed becomes nearly ion-depleted.
The acid compartment exhibits a very similar thermal profile, rising from 24.0˚C to 29.0˚C in the first 20 minutes, then to 33.0˚C at 40 min, and peaking at 36.0°C around 60–70 minutes. The acid loop warms primarily due to the high ionic mobility of H⁺ and the rapid accumulation of HCl, both of which increase conductivity while also contributing to resistive losses under constant voltage. The slight decline to ~34.0˚C in the later stage reflects the slowing of HCl production as Cl⁻ availability decreases and current drops.
The base compartment consistently exhibits the highest temperature among the three loops. The temperature increases from 25˚C at 0 min to 31˚C at 20 min, then to 34.5˚C by 40 min, ultimately reaching a peak of 36.5–37.0°C between 60 and 70 minutes. This elevated temperature relative to the acid and salt streams is attributable to the inherently higher resistance associated with OH⁻ transport (lower mobility compared to H⁺) and the increasing viscosity and ionic strength of the forming NaOH solution. These factors intensify Joule heating in the base compartment. Toward the end of the experiment, the temperature decreases slightly to 35.5˚C at 90 min, reflecting reduced current flow and lower ion transport rates as NaCl in the diluate becomes depleted.
Overall, the temperature evolution in all compartments reflects the interplay of ion availability, membrane transport resistance, solution conductivity, and current decline. The synchronized rise and moderate late-stage reduction in temperature confirm stable operation and predictable thermal behavior of the EDBM system during the electrodialytic conversion of NaCl into HCl and NaOH. As you can see in the figure given below.
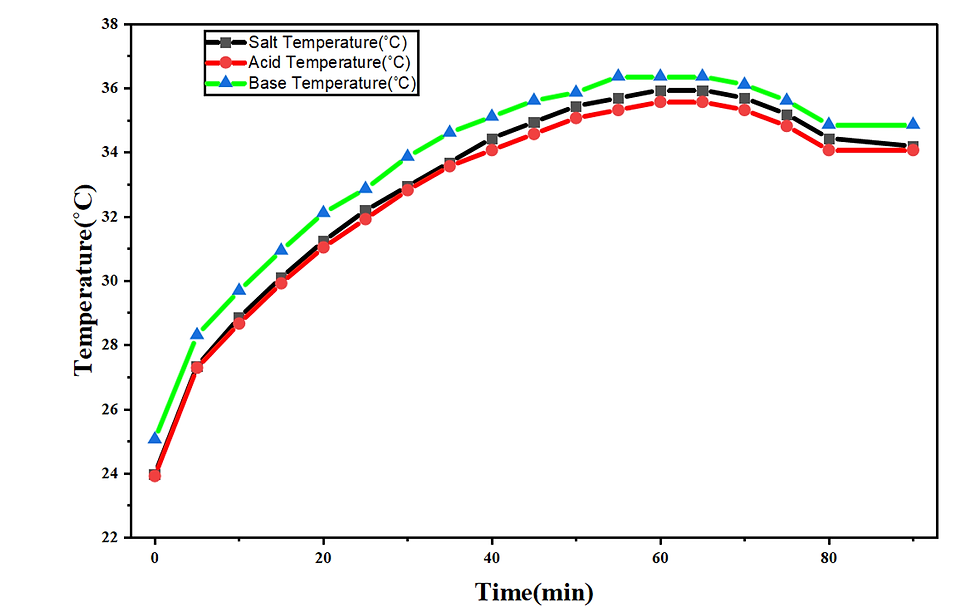
Figure 3: The temperature increase in the salt, acid, and base compartments throughout the electrodialysis operation, driven by Joule heating, ion transport resistance, and exothermic water-splitting reactions at the bipolar membrane interface, with the base compartment exhibiting the highest temperature due to the increased resistance associated with NaOH formation.
1.9. Current Vs Time graph
The current initially increases from 3.3 A at 0 min to 4.1 A at 10 min, reflecting the high ionic strength of the NaCl feed, which provides low resistance and allows rapid establishment of ion migration. As the experiment progresses to 30 min, the current reaches its maximum of approximately 5.1 A, indicating optimal membrane hydration, stable water splitting, and efficient transport of Na⁺ and Cl⁻ ions.
Beyond 30 – 40 min, the current begins to decline gradually from 5.0 A to 4.5 A, as NaCl starts depleting in the dilute compartment. This reduction in ionic availability raises electrical resistance and reduces charge transport efficiency. From 50 to 65 min, current decreases more sharply, dropping from 4.0 A to 2.1 A, aligned with increased resistance caused by the buildup of HCl and NaOH in the product compartments.
In the final stage, from 70 to 90 min, the current collapses from 1.1 A to 0 A, indicating near-complete depletion of NaCl and entry into a transport-limited regime where insufficient ions remain to sustain electrodialysis. As shown in the figure below.

Figure 4 The current-time profile during the electrodialysis operation, illustrating the rise, plateau, and eventual decline in current as ion transport stabilizes, and the feed solution becomes progressively depleted of NaCl, leading to a transport-limited regime.
1.10. Electrical Energy Consumption Vs Time graph
The energy consumption for the EDBM operation shows a steady and nearly linear increase from approximately 6.58 kWh at the beginning of the experiment to about 7.15 kWh at around 90 minutes. This upward trend reflects the progressive changes in electrical resistance and ion transport efficiency as NaCl is converted into HCl and NaOH.
At the start of the experiment (0 – 10 min), the energy consumption increases from 6.58 kWh to about 6.67 kWh. During this period, the salt compartment still contains a high concentration of Na⁺ and Cl⁻ ions, allowing efficient charge transport and relatively low resistance. The increase in energy during this initial phase corresponds to the establishment of stable current flow and initial water splitting at the bipolar membrane.
Between 10 and 40 minutes, the energy consumption rises from approximately 6.67 kWh to 6.90 kWh. This gradual increase is attributed to the decreasing ionic strength in the NaCl dilute stream as ions are continuously removed and transported to the acid and base compartments. As Na⁺ and Cl⁻ become less available, the electrical resistance of the dilute chamber increases, requiring more energy to maintain the applied voltage.
From 40 to 70 minutes, energy consumption continues to increase from 6.90 kWh to about 7.10 kWh. During this phase, the concentrations of HCl and NaOH in the product compartments rise significantly. The increasing ionic concentration and viscosity in these streams contribute to additional resistive losses. Furthermore, sustained water dissociation at the bipolar membrane requires increasing energy input as concentration gradients intensify.
In the final stage of the experiment (70 – 90 minutes), the energy consumption approaches a plateau around 7.14 – 7.15 kWh. This stabilization indicates that the system has reached a transport-limited regime. At this point, the NaCl in the feed is nearly depleted, resulting in reduced current flow but persistently high resistance, which maintains the overall energy requirement close to its upper limit.
Overall, the rise in energy consumption from 6.58 kWh to 7.15 kWh across the 90-minute duration reflects the combined effects of NaCl depletion, increasing membrane and solution resistance, and sustained water-splitting reactions at the bipolar membrane. As shown in the figure given below.

Figure 5: The energy consumption throughout the electrodialysis process, reflecting the gradual increase in energy as the system faces rising electrical resistance due to decreasing ionic strength in the NaCl feed and increasing concentrations of HCl and NaOH in the product streams.
1.11. Current vs. Energy Consumption
The relationship between current and energy consumption during the EDBM operation demonstrates a characteristic rise – plateau – decline pattern, reflecting the underlying changes in ion availability and membrane resistance as NaCl is converted to HCl and NaOH.
At the beginning of the process, when the energy consumption is low (≈6.58 kWh), the current is already moderately high at 3.3 A, due to the high ionic strength of the NaCl feed. As energy consumption increases slightly to 6.65 – 6.70 kWh, the current rises sharply to 4.1– 4.6 A, indicating efficient early-stage ion migration and minimal resistive losses in the membrane stack.
Between 6.70 and 6.90 kWh, the current reaches its peak range of 4.8–5.1 A, representing the operational period with optimal electrodialysis performance. In this region, Na⁺ and Cl⁻ concentrations in the diluate are still sufficiently high for effective transport, and water splitting at the bipolar membrane proceeds smoothly. This plateau region (current 5.0 –5.1 A) corresponds to the most productive phase of HCl and NaOH generation.
As energy consumption increases further to 7.00 kWh, the current begins to decline gradually from 4.9 A to 4.4 A, driven by the reduction in Na⁺ and Cl⁻ concentrations in the feed. The gradual increase in membrane and solution resistance results in lower current despite higher cumulative energy use.
A sharper decline occurs when energy consumption reaches 7.05 –7.12 kWh, where the current drops from 4.0 A to 3.1 A and continues down to 2.0 A. This steep downward trend indicates late-stage NaCl depletion and significant resistance buildup in the membrane stack.
Finally, as energy consumption reaches its upper limit of ≈7.15 kWh, the current falls to 1.1 A, and ultimately to 0 A, signaling complete ion depletion in the dilute compartment and the transition into a transport-limited regime. At this stage, the system no longer supports measurable ion migration, despite continued energy accumulation from the applied voltage. As shown in the figure below.

Figure 6: The relationship between energy consumption and current during the EDBM operation, depicting the U-shaped profile where energy consumption decreases during optimal ion transport and rises again due to increasing resistive losses as concentration gradients develop and ions are depleted.
Conclusion
This study successfully demonstrates the efficient conversion of NaCl into hydrochloric acid (HCl) and sodium hydroxide (NaOH) using the YASA ET pilot-scale bipolar membrane electrodialysis (EDBM) system equipped with a 10-pair Saifu EDBM stack. The pilot skid showed stable and predictable electrochemical behavior throughout the operation, confirming the robustness of the system design, the reliability of the membrane stack, and the suitability of the platform for acid–alkali production and process development.
The conductivity analysis revealed the characteristic signatures of EDBM operation: progressive depletion of NaCl in the dilute stream, with salt conductivity dropping from 105 mS/cm to nearly zero; rapid acid formation with conductivity rising to ~375 mS/cm; and steady NaOH generation reaching ~170 mS/cm. pH measurements aligned with these trends, showing strong acidification in the acid loop (pH 3.0 → 1.4), moderate acidification in the salt loop due to ion depletion (pH 3.3 → 2.0), and stable alkalinity in the base loop (pH ~12.2–12.4). These results confirm efficient water dissociation at the bipolar membranes and effective ion migration across the AEM, CEM, and BPM layers.
Temperature behavior was consistent with electrochemical expectations: all streams warmed steadily due to Joule heating, with the base stream reaching the highest temperatures (up to ~37˚C) because of increased resistance and higher NaOH viscosity. The current–time profile exhibited a typical rise–plateau–decline pattern, peaking at ~5.1 A during optimal ion transport and then falling to 0 A as NaCl became nearly depleted, marking the entry into a transport-limited regime. Energy consumption increased gradually from 6.58 kWh to 7.15 kWh, reflecting the cumulative resistive effects associated with decreasing salt concentration and increasing acid–base strength. The inverse and later direct relationship between current and energy consumption further confirmed the transition from high-efficiency to depletion-controlled operation.
Overall, the integrated analysis of conductivity, pH, temperature, current, and energy trends demonstrates that the YASA ET pilot EDBM system delivers stable performance, efficient ion separation, and effective acid-alkali generation under moderate voltage and controlled hydraulic conditions. The results validate the reliability of the Saifu and DESALT® membrane technologies for electrodialytic acid/base production and highlight the suitability of the pilot system as a development platform for scale-up to industrial EDBM units. This work provides a strong foundation for future optimization studies and industrial implementation of membrane-based acid–alkali recovery processes.




Comments