Sodium Chloride Concentration and Desalination Using a Pilot-Scale DESALT® Electrodialysis System: Performance and Energy Efficiency
- Gu Zhouying
- Dec 4, 2025
- 11 min read
Updated: Dec 16, 2025
Abstract
This study investigates sodium chloride (NaCl) concentration using a pilot-scale DESALT® electrodialysis (ED) system (YASA ET) under constant-voltage operation (14 V) in batch recirculation mode. The system, featuring a Saifu stack with ten cell pairs of alternating anion- and cation-exchange membranes, demonstrated efficient ion transport and strong membrane selectivity. The dilute stream conductivity decreased significantly from 54.6 mS/cm to 0.16 mS/cm, while the concentrate stream conductivity increased proportionally from 48.35 mS/cm to 139.29 mS/cm. Temperature rise in both circuits was moderate (≈26–29 ˚C) due to Joule heating and ionic friction. The current–time profile showed a decay from 2.1 to 0.1 mA·cm⁻², correlating with the increase in system resistance and ion depletion in the dilute compartment. Energy consumption increased linearly from 7.47 to 7.66 kWh per batch, driven by growing resistive losses and concentration polarization. This study highlights the relationship between current density, energy efficiency, and system resistance, providing insights for optimizing energy usage in electrodialysis desalination.
Keywords:
Electrodialysis, Bipolar Membrane Electrodialysis, NaCl Conversion, HCl Production, NaOH Production, DESALT®, Zero-Liquid-Discharge (ZLD), Acid-Base Generation, Pilot-Scale Study, YASA ET.
YASA Environmental Technology Co., Ltd.
No. 588, Xinjinqiao Road, Pudong, Shanghai, China
Email: info@yasa.ltd
Website: www.yasa.ltd
Introduction
The global challenge of water scarcity necessitates advanced separation technologies for the desalination of brackish and saline water sources, as well as for the treatment of complex industrial effluents. Among these technologies, electrodialysis (ED) has emerged as a mature and versatile electromembrane process. Its operational principle involves the selective transport of ions through alternating cation- and anion-exchange membranes under an applied electric field, thereby achieving desalination or concentration in respective compartments. Compared to pressure-driven processes like reverse osmosis, ED offers distinct advantages for specific applications, including high recovery rates, exceptional tolerance for feedwater with higher salinity or fouling potential, and the capability for selective ion separation.
The scalability of ED from laboratory prototypes to full-scale industrial plants, however, presents significant engineering challenges. While the fundamental electrochemical principles are well-understood, the practical performance is governed by a complex interplay of operational parameters, membrane properties, and fluid dynamics. Key factors such as current density, flow rate, and feed concentration directly influence critical outcomes, including ion removal kinetics, energy consumption, and the onset of limiting current density due to concentration polarization. These phenomena can only be accurately characterized at a pilot scale, where the hydraulics, membrane area, and stack design more closely mimic industrial systems.
Pilot-scale studies are therefore indispensable, serving as a critical bridge between foundational research and commercial implementation. They provide a robust platform for validating long-term membrane stability, optimizing energy efficiency, and generating reliable techno-economic data. A thorough investigation of the intrinsic relationships between voltage, current, and resistance under realistic conditions is paramount for predicting performance and controlling costs in larger-scale applications.
This study presents a systematic performance analysis of NaCl desalination using a DESALT Pilot ED unit. The primary objective is to elucidate the fundamental correlations between ion transport, evidenced by conductivity changes, and the resulting electrical and energy profiles under constant voltage operation. By monitoring the temporal evolution of current density and cumulative energy consumption, this work aims to quantitatively link the progressive ion depletion in the dilute stream to the increasing system resistance and energy demand. The findings provide critical insights into the operational dynamics of pilot-scale ED, contributing valuable data for the design and optimization of energy-efficient desalination and resource recovery processes.
Material and Methods
2.1. Electrodialysis System and Configuration
The experiments were conducted using a DESALT ED(BM) Pilot System (YASA ET), an advanced modular unit designed for both electrodialysis (ED) and bipolar membrane electrodialysis (EDBM). A comprehensive view of the pilot system, including the front operational panel and the rear plumbing and stack assembly, is presented in Figure 1. The core of the system is a Saifu electrodialysis stack, configured with ten cell pairs. Each cell pair consists of an alternating sequence of cation-exchange membranes (CEMs) and anion-exchange membranes (AEMs), separated by polypropylene spacers that define the flow channels. The effective membrane area for each sheet was 270 mm × 110 mm.


The ion-exchange membranes used were characterized by the following properties, as provided by the manufacturer: an ion-exchange capacity of 0.90-1.10 mmol/g, a wet thickness of 70-80 µm, and a water uptake of 20-25 wt.% at 25˚C, as shown in Table 1. The area resistance ranged between 4.5-5.5 Ω·cm², and the transport number exceeded 0.97, confirming high permselectivity. The membranes were stable across a broad pH range of 0-14 and operational temperatures of 15-40˚C.
Table 1. Characteristics of the ion-exchange membranes used in the ED stack.
Property | Value |
Ion-exchange capacity | 0.90 - 1.10 mmol/g |
Wet thickness | 70 - 80 µm |
Water uptake | 20 - 25 wt.% |
Area resistance | 4.5 - 5.5 Ω·cm² |
Transport number | > 0.97 |
pH range | 0 - 14 |
Temperature range | 15 - 40 ˚C |
2.2. Feed Solutions and Chemical Reagents
Aqueous sodium chloride (NaCl) solutions (ACS reagent grade) were prepared using deionized water to ensure high chemical purity and reproducibility. To establish stable initial conditions and minimize osmotic water transport across the membranes, both the dilute (feed) and concentrate compartments were initially filled with identical 35 g·L⁻¹ NaCl solutions. The electrode rinse solution (electrolyte) was formulated at a slightly higher concentration of 20 g·L⁻¹ NaCl, providing enhanced ionic conductivity within the electrode compartments. This configuration maintained a uniform and stable electric field distribution, reduced ohmic losses, and effectively mitigated parasitic voltage drops during electrodialysis operation.
2.3. Operational Procedure and Data Acquisition
The electrodialysis system was operated under constant-voltage conditions, with a direct current (DC) power supply maintaining a fixed potential of 14 V across the entire membrane stack. The volumetric flow rates of the dilute and concentrate circuits were precisely regulated at 70 L·h⁻¹ each using calibrated metering pumps, while the electrolyte loop was maintained at 40 L·h⁻¹. Each hydraulic circuit contained approximately 1 L of solution, which was continuously recirculated in batch mode throughout the experiment.
Before each experimental run, the complete system was flushed with tap water for 15–20 minutes to remove residual ions and subsequently rinsed with deionized water to ensure a clean baseline condition. The prepared NaCl solutions were then introduced into their respective compartments. A programmable logic controller (PLC) equipped with a Modbus communication interface facilitated automated operation and real-time data acquisition. The system continuously monitored pH, temperature, and conductivity across all process streams, while the applied voltage and resultant current were simultaneously recorded.
All process parameters were logged at five-minute intervals over a total experimental duration of 230 minutes, yielding a high-resolution dataset for performance evaluation. During operation, when the conductivity of the dilute tank decreased to 1 mS·cm⁻¹ or below, 35 g of NaCl was replenished into the dilute compartment to maintain continuous ion transfer. This cycle was repeated until the concentrate stream reached a maximum conductivity of 139.29 mS·cm⁻¹, indicating the highest achievable salt concentration under the applied conditions.
2.4.Electrodialysis Mechanism and Governing Reactions
The principle of electrodialysis relies on the selective electromigration of ions under an applied electric field. In a stack with alternating CEMs and AEMs, cations (e.g., Na⁺) migrate toward the cathode. They pass through CEMs but are blocked by AEMs. Conversely, anions (e.g., Cl⁻) migrate toward the anode, passing through AEMs but being blocked by CEMs. This selective transport results in the depletion of ions in alternate compartments (dilute) and the concentration of ions in the others (concentrate).
The overall process is driven by the electrochemical reactions at the electrodes. In a sodium chloride electrolyte, the primary reactions are:
At the Anode (Oxidation):
2Cl−→ Cl2(g) + 2e−
This reaction can be accompanied by the secondary reaction of water oxidation, especially at lower chloride concentrations:
2H2O → O2(g) + 4H+ + 4e−
At the Cathode (Reduction):
2H2O + 2e− → H2(g) + 2OH−
The protons (H⁺) generated at the anode and hydroxide ions (OH⁻) generated at the cathode are typically neutralized by the high-concentration electrolyte rinse stream, which prevents extreme pH shifts from damaging the stack components.
Results and Discussion
3.1. Ion Transport and Conductivity Dynamics
The temporal variation in electrical conductivity within the dilute and concentrated compartments provides clear quantitative evidence of ion transport dynamics during the electrodialysis process. As illustrated in Figure 2, the conductivity of the dilute stream decreased steadily from an initial value of 54.6 mS·cm⁻¹ to 0.16 mS·cm⁻¹ within the first 40 minutes of operation, corresponding to the period highlighted by the light blue rectangle. This pronounced decline reflects the efficient electromigration of Na⁺ and Cl⁻ ions from the dilute compartment through their respective cation- and anion-exchange membranes, thereby confirming effective desalination and charge-selective ion transport under the applied electric field.
Conversely, the concentrate stream exhibited a corresponding and proportional increase in electrical conductivity, rising from 48.35 mS·cm⁻¹ to 139.29 mS·cm⁻¹ over the 230-minute operating period. This complementary trend substantiates the fundamental electrodialysis mechanism, wherein ions are selectively removed from the dilute stream and accumulated within the concentrate compartment under the influence of the applied electric field. The initial stage of operation was characterized by a rapid increase in conductivity, reflecting a high ion flux driven by a strong concentration gradient across the membranes. As desalination progressed, the rate of conductivity changes gradually diminished, indicating the system’s approach toward quasi-steady-state ionic equilibrium, wherein the driving force for electromigration becomes increasingly counterbalanced by the back-diffusion of ions from the concentrate side.

3.2. Thermal Buildup from Joule Heating and Ionic Friction
A gradual temperature increase was recorded in both the dilute and concentrated circulation loops, as illustrated in Figure 3. The temperature of the dilute compartment increased steadily from an initial 26.22 ˚C to 28.84 ˚C during the first cycle with a 35 g·L⁻¹ NaCl solution, while the concentrate compartment exhibited a corresponding rise from 20.95 ˚C to 29.07 ˚C within the initial 40 minutes, as indicated by the large green rectangle in the figure. Notably, at the end of each batch, a sudden drop in the dilute temperature was observed (highlighted by the small light blue rectangles), corresponding to the addition of a fresh 35 g·L⁻¹ NaCl solution at room temperature. This thermal behavior primarily arises from Joule heating, wherein part of the applied electrical energy is dissipated as heat due to ohmic resistance (I²R losses) within the ionic solutions and the membrane stack. Additional minor contributions may originate from ionic friction and limited convective heat transfer within the recirculating loops.
Additional factors contributing to the observed temperature rise include the frictional resistance experienced by ions as they migrate through the membrane matrix, as well as the exothermic enthalpy changes associated with ion hydration and dehydration processes occurring at the membrane–solution interfaces. In the closed-loop recirculation system, where heat dissipation to the surroundings is limited, the generated thermal energy progressively accumulates, resulting in the steady temperature increase observed during operation. Such a thermal profile is a characteristic feature of electrodialysis systems, reflecting the inherent coupling between ionic transport, electrical resistance, and thermal energy generation under continuous electrochemical operation.

3.3. Current Decay as an Indicator of Increasing System Resistance
The current–time profile shown in Figure 4 exhibits a characteristic decay pattern, decreasing from an initial value of approximately 2.1 mA·cm⁻² to a final steady-state value of about 0.1 mA·cm⁻² during the first 40 minutes of operation, corresponding to the removal of 35 g·L⁻¹ NaCl, as indicated by the light blue rectangle. Multiple similar trends observed throughout the graph represent successive electrodialysis batches, each following a comparable decay behavior. This consistent pattern directly reflects the progressive increase in the system’s electrical resistance under a constant applied voltage, resulting from the gradual depletion of ionic species within the dilute compartment as desalination proceeds.
The current-time profile presented in Figure 4 demonstrates a characteristic decay behavior, declining from an initial value of approximately 2.1 mA·cm⁻² to a final steady-state level of about 0.1 mA·cm⁻² within the first 40 minutes of operation, corresponding to the removal of 35 g·L⁻¹ NaCl, as highlighted by the light blue rectangle. Successive cycles exhibit repetitive current decay trends, each representing an independent electrodialysis batch with similar electrochemical dynamics. This recurrent pattern reflects the progressive increase in the system’s overall electrical resistance under constant-voltage operation, primarily caused by the depletion of mobile ionic species in the dilute compartment as desalination advances.

3.4. Temporal Evolution of Energy Demand
Cumulative energy consumption, determined from the integration of real-time voltage and current data, exhibited a linear increase throughout the operational period, as illustrated in Figure 5. This linear relationship reflects the sustained electrical power input required to drive ion transport across the membranes as the concentration gradient between the dilute and concentrate compartments progressively intensified, resulting in higher resistive losses and increased energy demand over time.
At the onset of the electrodialysis process, the specific energy consumption per unit of salt removed was relatively low, indicating efficient ion transport under conditions of high conductivity and minimal electrical resistance. As the process progressed, concentration polarization developed, and the resistance of the dilute compartment increased, necessitating a greater electrical input to sustain the same potential difference and drive ionic migration across the membranes. Consequently, the cumulative energy consumption exhibited a steady linear increase, emphasizing the growing energy demand required to overcome these progressively unfavorable electrochemical conditions—an inherent efficiency limitation in batch-mode ED operations.
In the first batch, highlighted by the light blue rectangle in Figure 6, energy consumption increased from 7.47 kWh at the beginning to 7.66 kWh by the end of the run. This increment can be attributed to the declining ionic concentration in the dilute compartment: initially, a greater number of charge carriers facilitates low-resistance ion migration, whereas toward the end, fewer available ions result in higher electrical resistance and consequently greater energy expenditure to achieve equivalent ion transport.
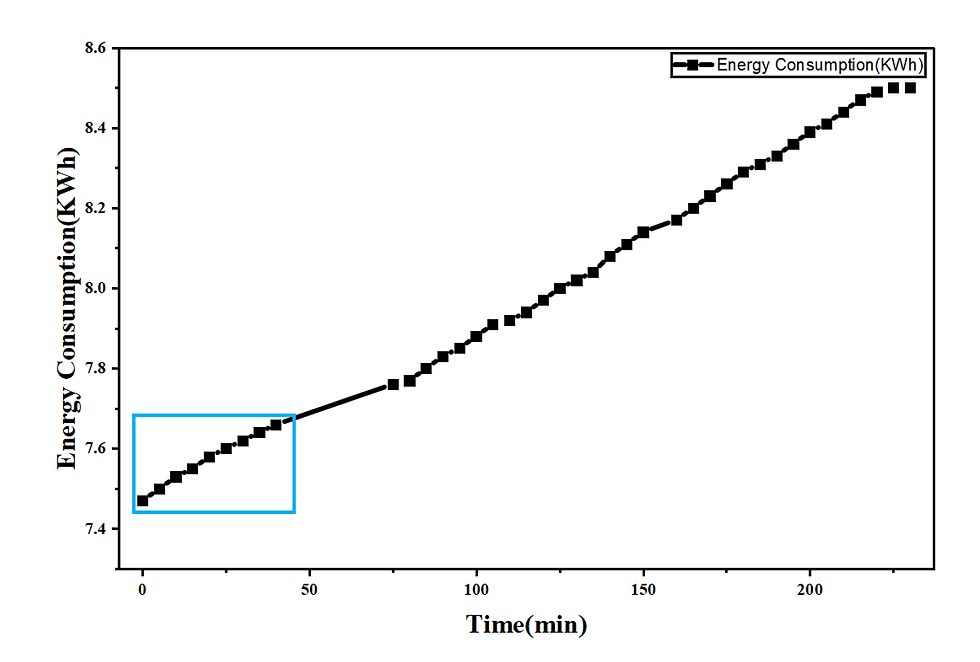
3.5. Interplay Between Operating Current and Energy Efficiency
Figure 6 illustrates the critical relationship between the operating current and the system’s energy efficiency. At the initial high current density of approximately 2.1 mA·cm⁻², the specific energy consumption, defined as the energy required per unit of salt removed, was measured at 7.5 kWh. This relatively low energy requirement reflects the efficient utilization of electrical input for ion transport under conditions of low system resistance and high ionic conductivity, where the majority of the applied power is effectively converted into productive ionic migration rather than dissipative losses.
As the process progressed and the operating current declined to approximately 0.2 A due to the increasing electrical resistance of the system, the specific energy consumption rose to 7.64 kWh, as shown for the first batch highlighted by the light blue rectangle in Figure 6. This inverse correlation between current and energy efficiency represents a fundamental characteristic of the electrodialysis process: as ionic depletion occurs within the dilute compartment, a greater amount of energy is required to transport each subsequent ion across the membranes. The data clearly demonstrate that lower current conditions, which coincide with higher degrees of desalination, impose a significant energy-efficiency penalty. This observation underscores the importance of process optimization, as in applications demanding high-purity product streams, the energy cost increases nonlinearly, emphasizing the need for strategies to minimize concentration polarization and associated resistive losses.

Conclusion
The pilot-scale electrodialysis experiments successfully validated the operational performance of the DESALT® ED(BM) system for NaCl concentration and desalination. The observed electrochemical behavior, including the conductivity decline in the dilute stream, current decay, temperature rise, and linear energy accumulation, collectively confirms the consistency of ion transport and the electro-thermal characteristics inherent to electrodialysis.
At the beginning of each batch, high ionic concentration and low electrical resistance enabled efficient ion migration with minimal energy loss. As desalination proceeded, the gradual depletion of charge carriers increased resistance and energy demand, leading to a steady decline in current and efficiency. The maximum concentrate conductivity of 139.29 mS·cm⁻¹ demonstrates the strong selectivity and stability of the membrane stack, while the moderate thermal buildup reflects efficient Joule energy dissipation control.
These results provide critical insights into the interdependence of current density, resistance, and energy consumption in pilot-scale ED systems. The findings emphasize that energy efficiency deteriorates under low-conductivity conditions due to polarization effects, highlighting the importance of optimizing operational parameters such as flow rate, voltage, and batch duration. The DESALT® pilot platform thus serves as a robust and scalable tool for advancing energy-efficient electrodialysis in industrial desalination and resource recovery applications.



Comments